QUICK REVIEW
PHYSICS
Physics is the branch of science that studies the nature and behavior of matter, energy, and the interaction between them
The main Branches of Physics
Physics is constituted of many interdisciplinary subjects and branches for those interested in
making a career in this field. Here are all branches of Physics:
1. Classical Physics
2. Modern Physics
3. Nuclear Physics
4. Atomic Physics
5. Geophysics
6. Biophysics
7. Mechanics
8. Acoustics
9. Optics
10. Thermodynamics
11. Astrophysics
INTERNATIONAL SYSTEM OF UNITS (SI)
.
| Basic quantity | Symbol of Basic Quantity | Name of the Base SI units | Symbol of SI units |
| Time | T | Second | s |
| Length | L | Metre | m |
| Mass | M | Kilogram | kg |
| Temperature | T, θ | Kelvin | K |
| Electric current | I | Ampere | A |
| Amount of substance | n | Mole | mol |
| Luminous intensity | lv | Candela | cd |
PREFIXES FOR SI UNITS
| Value | Prefix | Symbol |
| 1024 | yotta | Y |
| 1021 | Zetta | Z |
| 1018 | exa- | E |
| 1015 | peta- | P |
| 1012 | tera- | T |
| 109 | giga- | G |
| 106 | mega- | M |
| 103 | kilo- | K |
| 102 | hecto- | h |
| 101 | deca- | da |
| 10-1 | deci- | d |
| 10-2 | centi- | c |
| 10-3 | milli- | m |
| 10-6 | Micro | μ |
| 10-9 | nano- | n |
| 10-12 | pico- | p |
| 10-15 | femto- | f |
| 10-18 | atto- | a |
| 10-21 | zepto | z |
| 10-24 | yocto | y |
SUPPLEMENTARY UNIT:

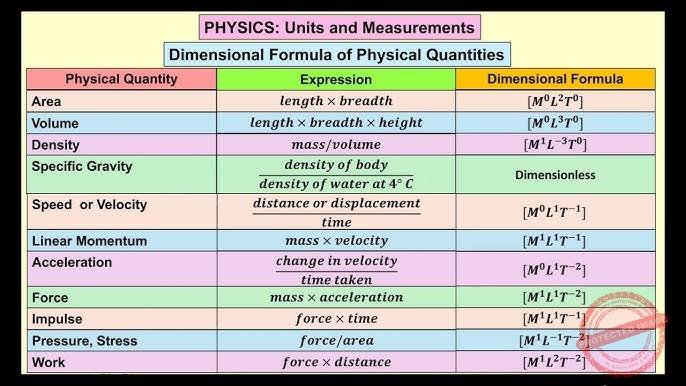
DIMENSION
The dimension of a physical quantity is the power of the fundamental quantities in terms of which it can be represented. Each of the basic quantities is called “ dimension” Or
The word dimension has a special meaning in physics. It is used to denote the nature of the physical quantity.
| DIMENSION | Symbol |
| Time | [T] |
| Length | [L] |
| Mass | [M] |
| Temperature | [T], [θ] |
| Electric current | [A] |
SIGNIFICANT FIGURES
The number of accurately known digit and first doubtful digit( called least significant)
are known as significant figures or significant digit.
RULES OF DETERMINATION SIGNIFICANT FIGURES
ALL non-zero numbers (1,2,3,4,5,6,7,8,9) are ALWAYS significant
EXAMPLE
- 3423 (ii) 457435
4- significant figure 6 -significant figure
(iii) 587 (iv) 58
3- significant figure 2- significant figure
ALL zeroes between non-zero numbers are ALWAYS significant
EXAMPLE
- 3003 (ii) 009
4- significant figure 5 -significant figure
(iii) 507 (iv) 5000097
3- significant figure 3- significant figure
Zeros locating the position of decimal in numbers of magnitude less than one are not significant
EXAMPLE
- 0063 (ii) 0.009
2- significant figure 1 -significant figure
(iii) 0.0000307 (iv) 0.0009078
3- significant figure 4 -significant figure
Final zeros to the right of the decimal point are significant.
EXAMPLE
- 0500 (ii) 0.00900
5- significant figure 3 -significant figure
(iii) 9.00300 (iv) 0.090200
6- significant figure 5- significant figure
Zeros at the end of the numbers greater than one may are may not be significant
EXAMPLE
- 700 (ii) 980000
1- significant figure 2- significant figure
For any value written in scientific notation as A × 10x, the number of significant figures is determined by applying the above rules only to the value of A.
EXAMPLE
4.5 × 103 has two significant figures
4.50 × 10-9 has three significant figures
4.500 × 1023 has four significant figures
COMBINING UNCERTAINTY: ADDING AND SUBTRACTING
Q-1 The length of a copper wire at 30°C is 18.2 ± 0.04 cm and at 60°C is 19.7 ± 0.02 cm. Find the absolute uncertainty and die extension of the wire.
DATA
The length L1 = 18.2 ± 0.04
The length L2 = 19.7 ± 0.02
SOLUTIONS
Extension of the wire
L = (L2 – L1 )
L = (19.7-18.2)
L = 1.5mm
Absolute uncertainty
∆L = ∆L1 + ∆L2
∆L = 0.04 +0.02
∆L = 0.06
Extension of the wire with uncertainty = ( L ± ∆L )
Extension of the wire with uncertainty = ( 1.5 ± 0.06 ) mm
Q- w = (4.52 ± 0.02) cm, x = ( 2.0 ± 0.2) cm, y = (3.0 ± 0.6) cm. Find z = x + y – w and
its uncertainty.
| DATA
w = (4.52 ± 0.02) cm x = ( 2.0 ± 0.2) cm y = (3.0 ± 0.6) cm SOLUTIONS Dz = Dx + Dy + Dw Dz = 0.2 + 0.6 + 0.02 Dz = 0.82 |
So z = x +y -w
z = 2.0 +3.0 -4.52 z = 5.0 -4.52 z = 0.48 cm Answer with uncertainty Z = (z ± ∆z ) cm Z = (0.48 ± 0.82) cm |
Q- Three objects have masses of 3 ± 0.1 kg, 7 ± 0.1 kg, and 4 ± 0.05 kg. What is the uncertainty in the total mass of the three objects?
| DATA
m1 = (3 ± 0.1 ) kg m2 = (7 ± 0.1 ) kg m3 = (4 ± 0.05) kg SOLUTIONS. m = m1 + m1 + m1 = m = 3 + 7 + 4 = 14 kg |
∆m = ∆ m1 + ∆ m2 + ∆ m3
∆m = 0.1 + 0.1 + 0.05 ∆m = 0.25 Answer with uncertainty = m ± ∆m = (14 ± 0.25 ) kg |

